The Controversy of Over- the- Counter Cough and Cold Medication for Children
 The reason for this started around October 2006, a group of chief pediatrics from the Baltimore Area, the Maryland Chapter of American Academy of Pediatrics, Dr. Janet Serwint of John Hopkins and Baltimore City Health Department all joined together to issue an advisory to parents for over the counter cough and cold medications for children ages five and under. In recent years doctors have reported serious injuries and death, and studies have failed to show effectiveness in children. The group petitioned to the commissioner of the FDA to provide a statement to the public explaining that over-the-counter antitussive, expectorant, nasal decongestant, antihistamine and combination cough and cold products have not been shown to be safe and effective for the treatment of cough and cold in children under six years of age. And that they notify manufacturers of these products whose labeling uses such terms as “infant” or “baby,” or displays images of children under the age of 6 that such marketing is not supported by scientific evidence and manufacturers will be subject to enforcement action at any time. The FDA should also amend 21 CFR 341 to require that labeling for over-the-counter antitussive, expectorant, nasal decongestant, antihistamine, and combination cough and cold products state that these products have not been found to be safe or effective in children under 6 years of age for treatment of cough and cold and that these products should not be used for treatment of cough and cold in children under 6 years of age.
The reason for this started around October 2006, a group of chief pediatrics from the Baltimore Area, the Maryland Chapter of American Academy of Pediatrics, Dr. Janet Serwint of John Hopkins and Baltimore City Health Department all joined together to issue an advisory to parents for over the counter cough and cold medications for children ages five and under. In recent years doctors have reported serious injuries and death, and studies have failed to show effectiveness in children. The group petitioned to the commissioner of the FDA to provide a statement to the public explaining that over-the-counter antitussive, expectorant, nasal decongestant, antihistamine and combination cough and cold products have not been shown to be safe and effective for the treatment of cough and cold in children under six years of age. And that they notify manufacturers of these products whose labeling uses such terms as “infant” or “baby,” or displays images of children under the age of 6 that such marketing is not supported by scientific evidence and manufacturers will be subject to enforcement action at any time. The FDA should also amend 21 CFR 341 to require that labeling for over-the-counter antitussive, expectorant, nasal decongestant, antihistamine, and combination cough and cold products state that these products have not been found to be safe or effective in children under 6 years of age for treatment of cough and cold and that these products should not be used for treatment of cough and cold in children under 6 years of age.
Retrieved October 16 2009, from FDA's Website:
www.fda.gov/ohrms/dockets/ac/07/slides/2007-4323s1-03-Petitioner-Sharfstein.ppt - 2007-11-15
Monday, October 26, 2009
Things to know before giving your child otc medications
Sunday, October 25, 2009
Alternatives to cough medication.
Voluntary Withdrawl of Infant Cough, Cold Medications by Drug Makers
 On August 15, 2007, the FDA issued a health advisory report warning parents not to give over the counter cough and cold medications to children under the age of 2. This recommendation is due to autopsy confirmed infant deaths directly related to complications from taking cough and cold medications. Following this discovery on October 11, 2007, the Consumer Healthcare Products Association (CHPA) announced on behalf of various drug maker and distributors the voluntary withdrawal of their over the counter infant cough and cold medications.
On August 15, 2007, the FDA issued a health advisory report warning parents not to give over the counter cough and cold medications to children under the age of 2. This recommendation is due to autopsy confirmed infant deaths directly related to complications from taking cough and cold medications. Following this discovery on October 11, 2007, the Consumer Healthcare Products Association (CHPA) announced on behalf of various drug maker and distributors the voluntary withdrawal of their over the counter infant cough and cold medications. The following is a list of branded infant cough and cold medication that were voluntary pulled from the market:
Little Cold Decongestant Plus Cough
Little Colds Multi-Symptom Cold Formula
PEDIACARE Infant Drops Decongestant & Cough
PEDIACARE Infant Dropper Decongestant
PEDIACARE Infant Dropper Long-Acting Cough
Robitussin Infant Cough DM Drops
Triaminic Infant & Toddler Thin Strips Decongestant Plus Cough
TYLENOL Concentrated Infants Drops Plus Cold
Saturday, October 24, 2009
Infant Deaths Assoicated with OTC cough and Cold Medicaitons in Infants.
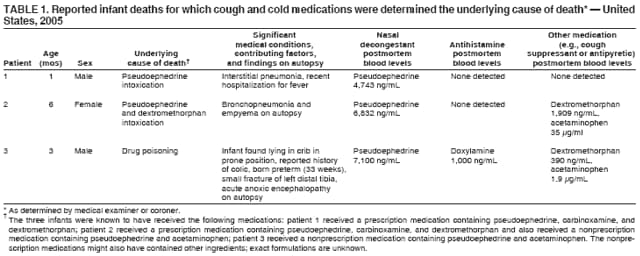
Cohen A, Shehab N, Srinivasan A.(2007 January 12).Infant Deaths Associated with Cough and Cold Medications--Two States 2005. MMWR weekly 56(01), 1-4. Retrieved from the MMWR website: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5601a1.htm
Friday, October 23, 2009
Parents just don't know.
Making Assumptions Could Cost Lives
Thursday, October 22, 2009
Myth or Fact?
Cough and cold are common problems among children due to viral infections. Many parents overlook the fact that when they walk to a local drug store and pick up an over the counter cough or cold medicine, they are overlooking the underlying reasons of why their children are having cough and cold? According to Kelly LK and Allen PJ, in their journal “Pediatric Nursing” many of those parents who choose over the counter cough and cold medications for their children need to rethink. According to them “the stated actions of some combination cough preparations are contradictory” and many cough medications are advertised as “multi-symptom”.
References
Wednesday, October 21, 2009
What is the medication industry doing to help decrease overdosing?
Physicians support the idea of FDA regulations on pediatric
Reference: